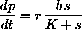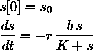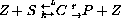
where S represents substrate, Z represents enzyme, C represents the substrate-enzyme complex, and P represents the product. PARAMETERS
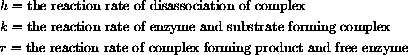
Each of the rate constants is proportional to the product of the concentration of the reactants, so
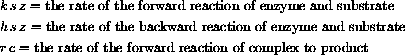
As a differential equation the enzyme-substrate forms and unforms by
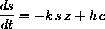
The reaction of complex to product is given by
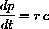
Three things change the concentration of complex. It can disassociate back into substrate and enzyme, it can react into product and enzyme, or substrate and enzyme can combine to form it:
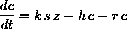
Since enzyme is either free and part of z or bound and part of c,
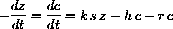
- 1. Prove that the sum a=s+c+p is constant. Hint: The derivative of a constant is zero.
- 2. Prove that b=z+c is constant.
- 3.
Use these relationship to prove that the four equations for
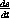 ,
, 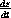 ,
, 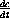 , and
, and
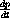 reduce to just the equations
reduce to just the equations
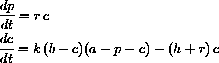
Explain how you would find z[t] and s[t] if you were given a solution to these two equations. - 4.
How are a and b determined at the beginning of a reaction? Write your answer as an augmented initial value problem,
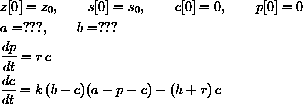
49.3
In some reactions, after a short period, the NET rate of formation of complex is small compared with the flow of substrate to complex and complex to product, so
This gives the Briggs-Haldane approximation:
Problem 49.1
Briggs-Haldane Numerical Experiments
Since the constant b=z+c, or c=b-z, and the rate of reaction forming product is
Ask your local neighborhood biochemist for some real examples where the Briggs-Haldane and Michaelis-Menten approximations apply.
Specifically, could k<<h when h>>r? Will c remain nearly constant for most of a reaction even when r, h, and k are comparable, but z is small?
Mathematically, you can explore the differences:
Problem 49.2
Michaelis-Menten Numerical Experiments
49.5
Blood alcohol (ethanol) at high concentrations like 100 millimolars causes vomiting and inability to walk.
Alcohol is metabolized by an enzyme called "alcohol dehydrogenase." The constant K for this enzyme is about 3 millimolars.
Problem 49.3
49.6
Carbon dioxide (CO2) is closely related to blood pH (acidity). There is an approximately constant concentration of CO2 in the blood of about 1.35 millimolars.
One mechanism that keeps this level constant is a reaction with an enzyme caled "carbonic anhydrase." The constant K for this enzyme is about 12 millimolars.
The average concentration of the enzyme is about 0.001 and the rate constant is on the order of 106 per second, one of the largest enzyme rate constants.
Problem 49.4
A Visit to the Chemistry Library
Find an intermediate speed reaction where Michaelis-Menten dynamics lie between hockey sticks and exponentials.
 .
.
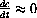 , show that
, show that
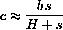
where H=(h+r)/k. (Hint: Use the constant b and the equation for the change in c.)
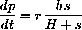
since
 constant.
constant.
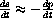 .
.
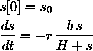
Compare the dynamics of the solution of the full enzyme dynamics in Hint 49.2.1 with the Briggs-Haldane initial value problem in the case where h=?, k=?, r=? and in the case h=??, k=??, r=??
A LIMITING CASE OF MICHAELIS-MENTEN DYNAMICS
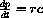 , the maximal rate of production is
, the maximal rate of production is
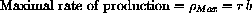
Also, in general,
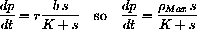
In real kinetic experiments, scientists plot
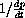 versus 1/s to measure the constants.
versus 1/s to measure the constants.
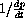 varies linearly with 1/s,
varies linearly with 1/s, 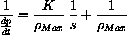
and explain how you could measure K from an experimental graph of
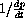 versus 1/s.
versus 1/s.
How do the Briggs-Haldane and Michaelis-Menten approximations compare? For example, under the Michaelis-Menten assumptions, how do the constants
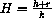 and
and
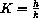 compare? Under the Michaelis-Menten assumptions, can the Briggs-Haldane assumptions also hold? What about the converse?
compare? Under the Michaelis-Menten assumptions, can the Briggs-Haldane assumptions also hold? What about the converse?
Compare the dynamics of the solution of the full enzyme dynamics in Hint 49.2.1 and the Briggs-Haldane approximation with the Michaelis-Menten initial value problem in the case where h=?, k=?, r=? and in the case h=??, k=??, r=??
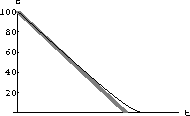
Figure 49.1: Sobering up for 4 Hours
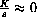 for most of the decay of a substance, show that
for most of the decay of a substance, show that
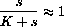
so that the differential equation is initially approximately
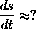
with solution s[t]=s0-rbt.
Look up the "linear" sobering rate for blood ethanol and adjust the constants above appropriately.
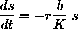
Why?
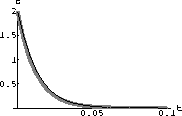
Figure 49.2: CO2 Concentration in the Blood
Find more exact values of the constants for the carbonic anhydrase reaction.
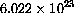 , of molecules of the substance.
This is the atomic weight of the compound in grams, so, for example, a mole of carbon 12 weighs 12 grams.
We will use units of "molars" or moles per liter in our examples, specifically millimolars.
The reason this choice of units is best is that one molecule of enzyme combines with one molecule of substrate to form one molecule of complex.
, of molecules of the substance.
This is the atomic weight of the compound in grams, so, for example, a mole of carbon 12 weighs 12 grams.
We will use units of "molars" or moles per liter in our examples, specifically millimolars.
The reason this choice of units is best is that one molecule of enzyme combines with one molecule of substrate to form one molecule of complex.


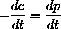
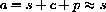 . We always have b=z+c, constant, so z=b-c.
. We always have b=z+c, constant, so z=b-c.
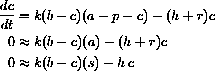
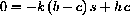
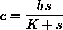
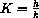 .
.