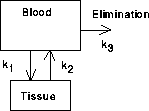
Figure 35.1: two-compartments
Differential equations are useful in studying the dynamics of a drug in the body. The study of such dynamics is called "pharmacokinetics." Here is a basic example. Suppose a drug is introduced into the blood stream, say by an intravenous injection. The injection rapidly mixes with the whole blood supply and produces a high concentration of the drug everywhere in the blood. Several tissues will readily absorb the drug when its concentration is higher in the blood than in the tissue, so the drug moves into this second tissue "compartment." If this were the whole story, the concentration would eventually balance out so that the concentration in blood and tissue were both equal to the total amount of drug divided by the total volume. However, this usually is not the end of the story.
The kidneys remove the drug from the blood at a rate proportional to the blood concentration. This causes the blood concentration to drop, and eventually it drops below the tissue concentration. At that point, the drug flows from tissue back into the blood and is continually eliminated from the blood by the kidneys. In the long term, the drug concentration tends to zero in both blood and tissue. The speeds with which these various things happen is the subject of pharmacokinetics.

Why should we care about such dynamics? Some drugs have undesirable, or even dangerous, side effects if their concentration is too high. At the same time those drugs must be above a certain concentration to be effective for their intended use. As the drug is eliminated from the body, doses need to be given periodically in order to maintain the threshold level for effectiveness, yet doses cannot be too frequent or too large or the concentration will exceed a dangerous level.
This project studies the basic dynamics of the two-compartment drug model. Projects 15 and 16 study some important consequences of these dynamics. The "two-compartment model" corresponds to the previous story. More complicated dynamics might include an intramuscular injection that first diffuses into the blood stream, then into perfuse tissue, and finally is eliminated by the kidneys. Another dynamic might be a drug that is metabolized while it is in the tissue compartment.
The actual "compartments" can vary according to the drug. For example, "blood volume" often includes highly perfused lean tissue such as the heart, lungs, liver, kidney tissue, endocrine glands and occasionally the brain and spinal system (since these often present different barriers to drugs). In these cases, "tissue volume" consists of muscle and skin. Another possible "compartment" is the fat group, adipose and marrow. The anticoagulant warfarin (used as a blood thinner and in rat killer) becomes bound to a protein in the blood. It is not absorbed in tissue, but the bound state forms a second "compartment."
For now we just consider a drug that is introduced into the blood, diffuses into, tissue and is eliminated by the kidneys. Some of the drugs that fit the two-compartment model are aspirin (acetylsalicylic acid); creatinine, a metabolite of creatine produced by muscle contraction or degeneration; aldosterone; griseofulvin (an antifungus drug); and lecithin.
PRIMARY VARIABLES OF THE MODEL
The primary quantity we wish to measure is the drug concentration in the blood.
That concentration is a function of time and is quickly and directly affected by the concentration of the drug in the tissue.
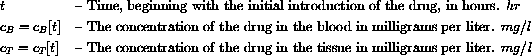
Each drug and each patient have certain important constants associated with them. In the complete story these will have to be measured.
PARAMETERS OF THE MODEL
Concentration is an amount per unit volume.
Suppose that a patient has 2.10 liters of blood and that 0.250 grams of a substance is introduced into his blood.
When mixed, the concentration becomes
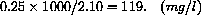 .
.
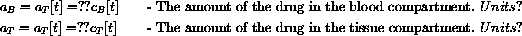
Suppose a patient has vB=2.10 and vT=1.30. If cB[t] and cT[t] were known functions, what would the amounts aB[t] and aT[t] be?
initConcs = Table[{c,0},{c,0,100,5}]; numgraphs = 10 ;flow2D[ {f,g}, {cB,cT}, initConcs, tfinal, dt, numgraphs ];
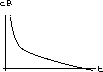
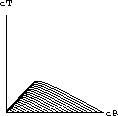
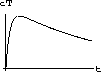
Explain intuitively why this equilibrium must be an attractor.
Notice that our concentration equations have the form of a linear system

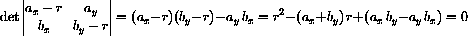
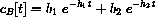
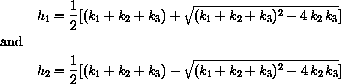

What we know from the theory is that

Reasonable approximations for b1 and b2 are easy to guess in the case h1>>h2. If the fast absorption into the tissue is so fast that the kidneys do nothing during this period, we know that the solution looks like
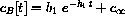 where
where
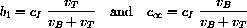
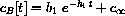 , for the constants above.
What are the necessary assumptions? (Hint: See a previous exercise.)
, for the constants above.
What are the necessary assumptions? (Hint: See a previous exercise.)
Show that k1>>k3 implies that h1>>h2 for some reasonable meaning of >>. (If you want to be formal, take k1 (and k2) positive but not infinitesimal and
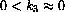 . Show that
. Show that
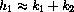 , while
, while
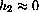 . Connect your formalization with the real problem.)
. Connect your formalization with the real problem.)
In the case where k3=0, the full solution could be written
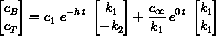
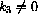 can be written in the vector form
can be written in the vector form
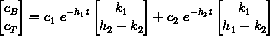
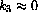 , the two solutions immediately before this exercise are close to one another for finite time.
, the two solutions immediately before this exercise are close to one another for finite time.
If we let
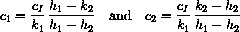
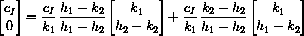
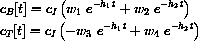
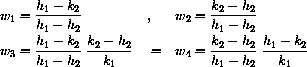
These formulas give the exact symbolic unique solution of the full drug dynamics equation with initial condition (cI,0)
What happens when k3=0? What happens when k3 is nearly as large as k1?
k1 = 0.17;
vB = 2.1;
vT = 1.3;
k3 = 0.03;
k2 = k1 vB/vT;
h1 = ((k1 + k2 + k3) + Sqrt[(k1 + k2 + k3) 2 - 4 k2 k3])/2;
2 - 4 k2 k3])/2;
h2 = ((k1 + k2 + k3) - Sqrt[(k1 + k2 + k3)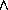 2 - 4 k2 k3])/2;
2 - 4 k2 k3])/2;
u1 = vT/(vT + vB)
w1 = (h1 - k2)/(h1 - h2)
u2 = vB/(vT + vB)
w2 = (k2 - h2)/(h1 - h2)
cB[t_] := cI (w1 Exp[ -h1 t] + w2 Exp[ -h2 t]);
cB[t]
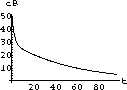
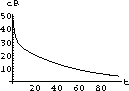
35.2
The exact symbolic solution to a single dose [or initial conditions (cI,0)] given in the previous exercise is the starting point for the Project 15. That project explores things like the peak tissue concentration, the time interval during which the concentration remains effective, and the total integral of effective concentration.
You could go to that project now to round out your thinking on pharmacokinetics.
Project 16 uses this single-dose symbolic solution and some basic work on logs from Project 15 to show how drug concentration data can be used to measure the rate parameters of this model.
You might go to that project now instead of working Project 15.
Other alternatives for completing this project are to study more complicated dosing regimens.
Usually a single dose of a drug is not given, but rather doses are either given periodically or a constant flow of drug is maintained in an intravenous fluid.
The analysis of these two regimens follows as your third and fourth alternatives.
Finally, a fifth alternative completion of the project is to study dosing by intramuscular injection.
In this case, the drug must diffuse from the muscle into the blood before the two-compartment dynamics takes effect.
This amounts to a three-compartment model.
35.3
Suppose a patient is given a first dose of a drug, a1 (mg) at time t=0. Typically, this dose is larger than subsequent doses.
After a time interval
At time
Also, the concentration in the tissue is not zero at time
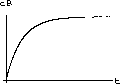
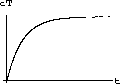
As an example, let us take a1=200 (mg) and a2=100 (mg) using the parameters k1=0.17, vB=2.10, vT=1.30, k3=0.03, as before.
The values of concentration before the second dose are cB[6]=52.6 and cT[6]=49.7.
This makes the new initial value problem beginning with the second dose
We can put this into AccDEsol as a new initial value problem and solve for six more time units, then repeat the process.
Or we can find the exact symbolic solution to this initial value problem and compute the concentrations six units later, add the third dose and continue with that procedure.
In either case we get the long-term drug dosages and the question is:
Will the concentrations build up? What is the long-term max? The min?
That's your project.
Here's some help if you want to use exact solutions which aren't really any better than numerical ones from AccDEsol.
The general solution to the differential equations may be written in the vector form
e2 = 49.7;
{c1,c2} = LinearSolve[{{k1,k1},{h2 - k2 , h1 - k2}},{e1,e2}]
cB[t_] := c1 k1 Exp[ -h1 t] + c2 k1 Exp[-h2 t];
cT[t_] := c1 (h2 - k2) Exp[ -h1 t] + c2 (h1 - k2) Exp[-h2 t];
cB[6]
cT[6]
Our calculations with the parameters and doses above gave the following:
35.4
The previous problem started with an initial dose of 200 mg and then followed that with doses of 100 mg every 6 hours. What would happen to the patient's drug concentrations if we constantly fed the drug into the blood stream at the rate of 100/6 = 16.7 mg/hr? This is much easier for us to analyze , because the "forcing term" or dosage is continuous.
First, we need to modify the basic equations,
Now we have an additional term of a constant growth r added to the blood concentration.
In the case described in the previous paragraph, r = 16.7/vB = 7.94 mg/l/hr. In general, this constant dose rate makes our initial value problem:
35.5
Our final example of dosing is an intramuscular injection.
In this case the drug is injected in high concentration in a muscle and then the drug diffuses into the blood.
Once in the blood, it diffuses into the tissue compartment and is eliminated by the kidneys.
Use the computer to solve this system with vM=0.250 and k4=1.5 and the parameters from the previous examples.
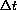 , say 6 hours, a second dose a2 is administered.
We know that the exact solution from time t=0 to
, say 6 hours, a second dose a2 is administered.
We know that the exact solution from time t=0 to
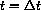 is given by the exponential sum,
is given by the exponential sum,
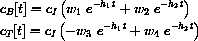
where cI=a1/vB and
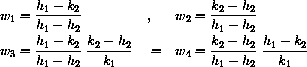
or we could simply use AccDEsol to find the solution.
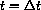 , the blood concentration is increased by a2/vB, but the old drug is not absent from the blood (and we often want to be sure it does not drop below a threshold of effectiveness). Say the concentration just before the second dose is
, the blood concentration is increased by a2/vB, but the old drug is not absent from the blood (and we often want to be sure it does not drop below a threshold of effectiveness). Say the concentration just before the second dose is
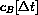 (using the formula above for cB[t].) Our new initial concentration becomes
(using the formula above for cB[t].) Our new initial concentration becomes
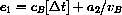 .
.
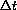 . We can use the formula above to compute
. We can use the formula above to compute
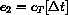 . As a result, we want to solve the new initial value problem
. As a result, we want to solve the new initial value problem
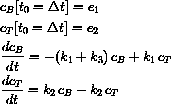
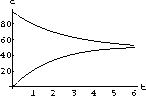
Figure 35.3: Concentrations up to Time 6 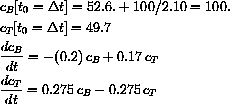
Notice that the initial value of cT is no longer zero.
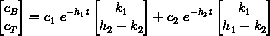
If we reset our clock at the second dose, we want to have

Notice that substitution of t=0 into the general vector form yields
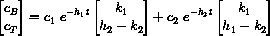
or in matrix form
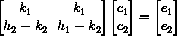
For example, these equations can be solved with Mathematicaby typing
e1 = 100.;
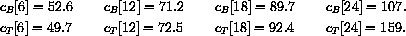
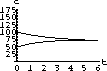
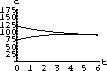
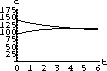
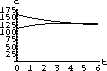
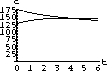
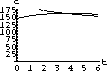
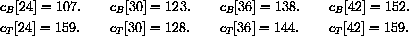
What are the concentrations at the end of 48 hours, just before the ninth dose? Do the concentrations continue to build up over time, or do they reach some maximum level and stop growing?
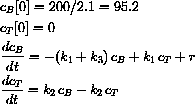
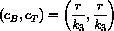
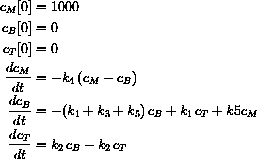
in terms of the intramuscular injection.
What is the amount of drug in the injection?